Most vaccines are in use for decades, with several individuals receiving them safely each year. Like all medicines, every vaccine should bear in-depth and rigorous testing to make sure it’s safe before it may be introduced in an exceedingly country’s vaccine program.
Every vaccine beneath development must initially undergo screenings and evaluations to work out that substance ought to be wont to invoke an immune response. Then the vaccine is tested on an animal afterwards on a human.
If the vaccinum triggers an immune response, it’s then tested in human clinical trials in 3 phases.
Phase One
The vaccine is given to a little range of volunteers to assess its safety, ensure it generates an immune response, and confirm the correct dosage. Usually during this phase vaccines are tested in young, healthy adult volunteers.
Phase Two
The vaccine is then given to many hundred volunteers to more assess its safety and skill to come up with an immune response. Participants in this phase have similar characteristics (such as age, sex) because of the people for whom the vaccinum is intended. There are usually multiple trials during this section to judge numerous age teams and different formulations of the vaccine.
Phase Three
To work out if the vaccinum is effective against the unwellness it’s designed to guard against and to review its safety in an exceedingly abundant larger cluster of people. Most of the time section 3 trials are conducted across multiple countries and multiple sites among a rustic to assure the findings of the vaccine performance apply to several different populations.
Discovery – Mistreatment Of The Isolated Infectious Agent To Develop A Potential Vaccine
Each disease has different characteristics, thus every vaccine can have a private development path. Betting on the disease in question, pathogens will either be reduced in efficiency or fully inactivated. This makes them safe to be used within the vaccines. In some cases, parts of the pathogens may be sublimated and combined. The addition of specialized molecules – referred to as adjutants – can then promote the body’s immune response.
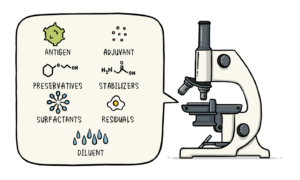
Pre-Clinical Testing – Understanding How The Potential Vaccine Works, And The Way It Probably Affects The Body
Before any testing in humans, a brand new vaccine can bear careful investigation in the laboratory, followed by trials in animals. The potential vaccine will be checked to make sure any purification has not altered its identity, which stimulates the suitable immune response. Impacts the results of adding any adjuvant system also will be valued. Animal trials follow strict tips set down by the restrictive authorities, and can solely be allotted once queries cannot be answered with another method.

Clinical Trials – Ending Tests In Humans
Testing in humans is conducted in 3 phases, all ruled by internationally united principles. In section I, studies on tiny teams of volunteers evaluate safety, immune effect, and tolerance to different doses. Phase II involves a bigger cluster and confirms formulations and doses, and identifies the necessity for boosters and also the best intervals between every dose. Clinical tests can value the protection given to {many} thousand volunteers who are in danger from the unwellness in question. Clinical trials will take many years.
Restrictive Approval – Submitting Information And Data To Regulators To Realize Approval For Vaccines
All the data and data collected throughout the development and trials of a brand new vaccine are bestowed to relevant regulators at the regional level (where appropriate, for instance within the EU), and national level. Before authorization is granted, all queries raised by the regulators must be answered that ensure consistency of standards.
Supplying, Producing, And Shipping – Delivering Vaccines To People Who Want Them
Just like the development of vaccines, manufacturing is very advanced and takes time. One specific challenge is that providing meets demand, which can usually mean the new vaccine has to be made in massive quantities.
Observation – Evaluating Vaccines Once An Introduction
Once a vaccine has been introduced to the market, it continues to be closely monitored at totally different levels. This ensures that the expertise gathered from its use in massive numbers of the population is captured, and its in-progress safety and effectiveness are closely assessed.
Conclusion
Vaccines are developed, tested, and controlled in a {very} very similar manner to different medications. In general, vaccines are even additionally tested than non-vaccine drugs as a result of the number of human subjects in vaccine clinical trials being sometimes greater. In addition, post-licensure observation of vaccines is closely examined by the Centers for unwellness management and also the FDA.